Professional and high-tech services
Strong guarantee for high-quality Clinical Trial data
Modeling and Simulations
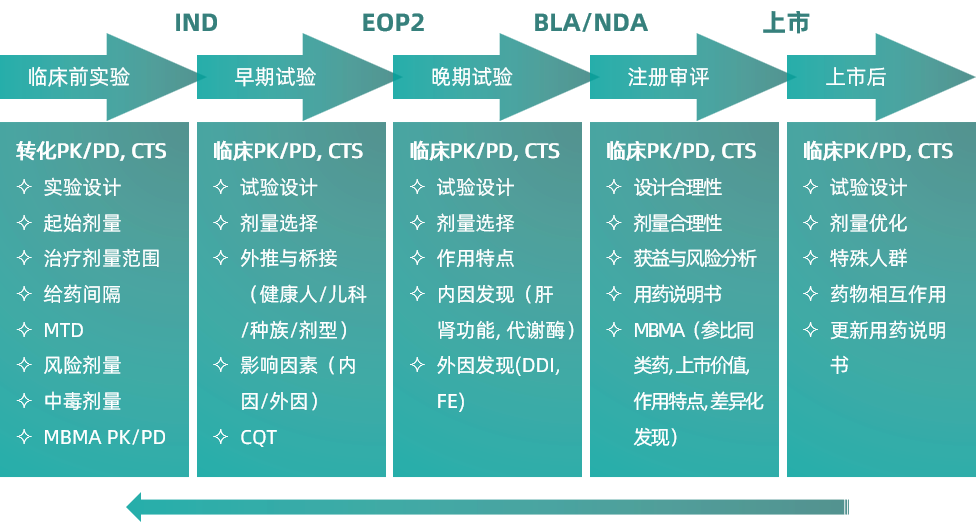
The application of quantitative pharmacology can greatly improve the efficiency and success rate of new drug research and development, and has become a key technology for new drug research and scientific evaluation.
We have a professional and stable research team, led by renowned quantitative pharmacology experts, and have been committed to the theoretical research and practice of the "Model Guided Drug Development (MIDD)" model for a long time, such as translational medicine, PBPK, FIH, risk dose control, treatment dose range determination, etc., to make early clinical trials more accurate. We have been applied in chemical drugs, biological products, including oncolytic viruses, monoclonal antibodies We have accumulated rich practical experience and successful cases in various categories of drugs such as traditional Chinese medicine; We have accumulated rich practical experience and successful cases in modeling and simulating popPK/PD, PK, and/or PKPD guided dose escalation (precise climbing), dose optimization, MBMA determination of standard efficacy scales, differentiated research and development, racial difference analysis, Chinese and foreign data bridging, adult extrapolation of children, etc.
Service Content
R&D strategy development and consulting
Population Pharmacokinetic Analysis (popPK)
Population Pharmacokinetic and Pharmacodynamic Model Analysis (popPK/PD)
E-R Analysis
Physiological based pharmacokinetic model (PBPK)
C-QTc Analysis
Translational Medicine analysis
MIDD研发模式