DaS PVS
It is a fully self-developed, professional and standardized intelligent platform for drug safety management tailored for Sponsor/CROs.
It integrates data collection, query management, data analysis, medical coding, automatic reporting, Document retrieval, and data interaction, and can be configured quickly and flexibly. It is committed to the establishment of enterprise safety database and helps to standardize the construction of China's pharmacovigilance system.

Features
| Initial case registration, case duplication check
| Case report and follow-up management, supporting one click follow-up report generation and case replication
| Report Manual numbering and Automatic numbering of combination condition system (self defined numbering rules)
| Customized workflow, process submission, task, message notice
| E2B (R3) rule verification, form and file verification
| Automatic narrative generation, supporting custom template creation and referencing
| Visualization of medical review, clearly timeline view
| Medical coding: support MedDRA and WHO Drug medical dictionaries; Code review and proofreading;
| Literature reports, support regular retrieval, and implement retrieval; Document processing markers
| 定期报告管理,自动创建、提醒;报告撰写、审阅、定稿
| Automatically reports in various forms such as CIOMS, ICSR, XML, etc
| Gateway docking and multi form report submission
Case Report
Task activities
Query Mgt
Case Narrative
Literature Retrieval
Aggregative Report
Study Mgt
Product License
Line-Listing and data export
EDI Gateway
Medical Coding
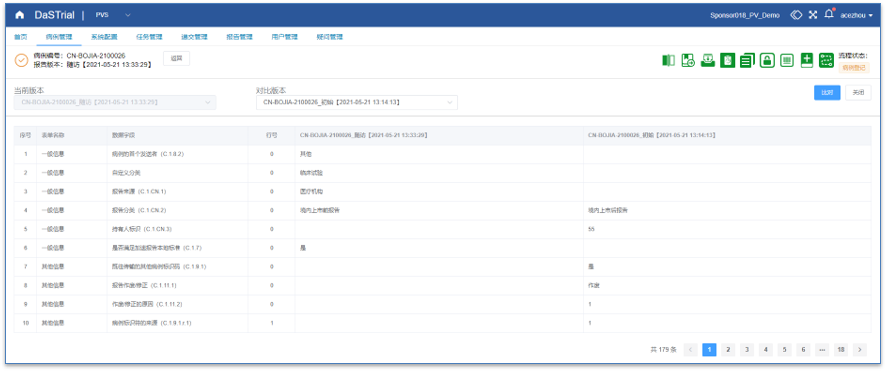
Medical Review
Intuitive Medical Review
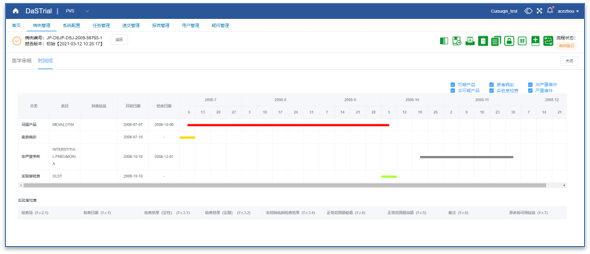
Easier Medical Coding
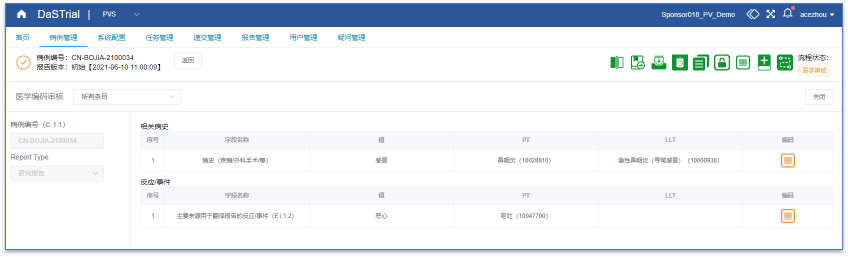
Controllable follow-up reports