
DaS ePRO
DaS ePRO system provides a flexible and efficient solution for remote collection of clinical trial data; The data required for clinical trials can be easily, accurately, and completely collected through mobile devices. Unlike other independent ePRO systems, the EDC based ePRO integrated system architecture can eliminate the management issues of multiple independent systems and achieve the advantages and value of platformized data management, better than other systems.
System advantages
- Platformized management: EDC database serves as a unified data storage, and ePRO independent interface is used for management;
- Multi environment: five default environments, data independence and security; Can be used for different purposes such as training, testing, acceptance, production, etc; Interconnection with EDC;
- Custom role permissions: ePRO platform users can achieve different roles in different sites and environments;
- Subject synchronization: You can choose EDC or directly add new subjects in the ePRO system, with data synchronization at both ends;
- Multi end access: Participants log in and fill out tasks through the WeChat app, with stronger compatibility; Site users can log in to the platform system through WeChat mobile phones and computers;
Flexible diary configuration and setup
Relying on DaS EDC data structure design, ediary forms can be flexibly and personalized to meet all needs, whether it is any form of scale or custom data point collection;
Edit Check verification
With the powerful Edit Check functions of EDC, Data issues can be quickly and accurately identified, ensuring the compliance of subject data;
Auto task push
Task notice to improve subject compliance and accurately obtain subject data; At the same time, providing spontaneous data filling is more flexible and simple.
Audit History
All subjects operational record to ensure traceability and meet regulatory requirements.
100%Data Integration
Using Both DaS EDC and ePRO, there is no need to enter data repeatedly, greatly facilitates the data management process, also can find data issues in time to obtain high-quality data;
Convenient data view for expanding
Convenient data viewing interface, all EDC function extensions (report export), and more data management methods;
Alert Notice
Support multiple notice (WeChat, email). If subject's indicators are above the warning limit, actively remind the site personnel to ensure the safety of the subjects.
Scale plugin
Provide specific scale plugins that are more suitable for practical clinical operations and facilitate subject evaluation reports.
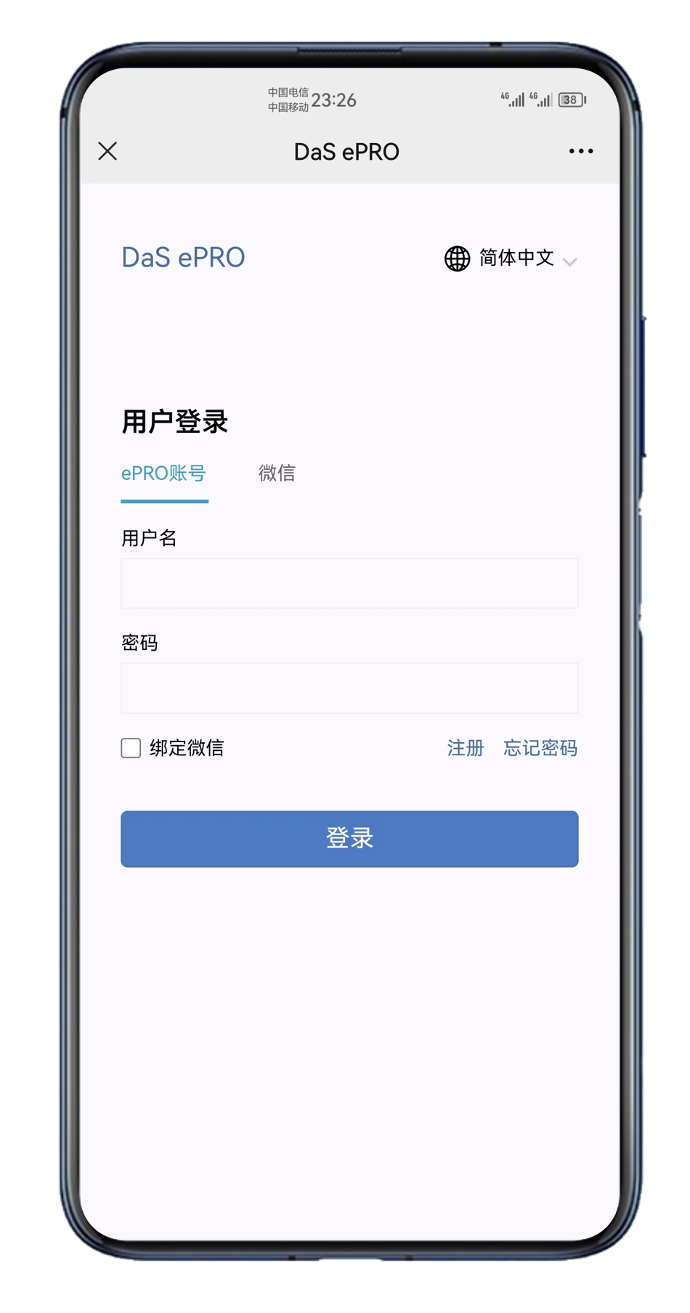
- User account login, WeChat account binding, easy to operation; Support for multiple account switching;
- Platform registration; Mobile verification;
- Customized plan arrangement for subjects; Automatic scheduling; Manual scheduling;
- Spontaneous reporting, real-time and spontaneous reporting for AE and CM;
- To view the filling history, investigators can directly view the participant's filling records in the EDC system;
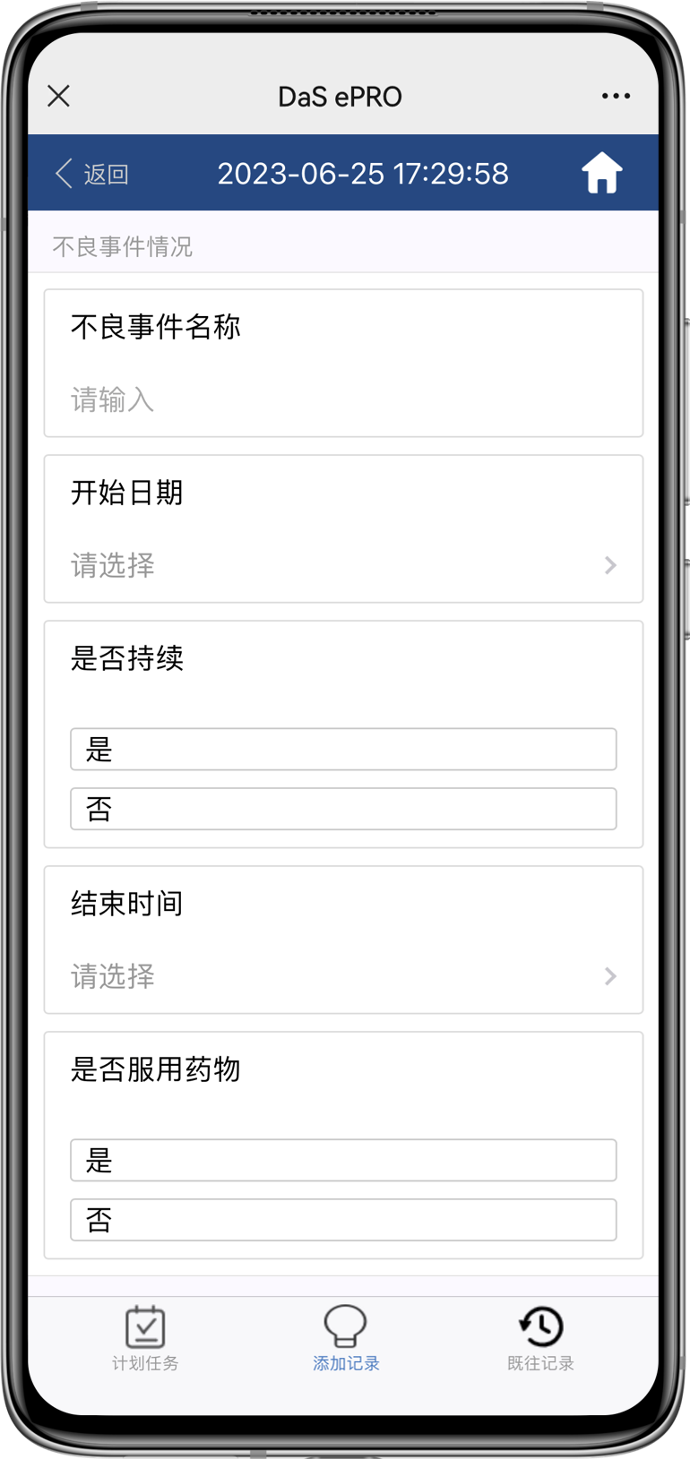
EDC real-time data verification with configurable Alert
- Subject filling data derectly into the EDC system
- Edit Check real-time verification
- Optionnal to merge with EDC database or alone ePRO database
- EDC system access control, precise to field variables
- Real-time data alerts through EDC notice, notify investigator of exceeding thresholds, and ensure subject safety