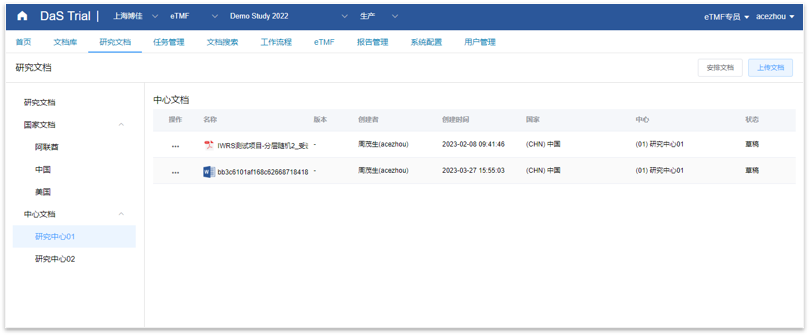
DaS eTMF
DaS eTMF is a document management system designed specifically for the clinical trial industry. It is a cloud platform system based on internet cloud technology, which can perfectly support DIA RM, customize TMF RM, and achieve the full process management of document writing, transmission, review, approval, and archiving during clinical trials; At the same time, it complies with GCP and regulatory standards, with all operational records and audit trails, and effectively and simply manages enterprise level documents.


Tasks and workflow
Document lifecycle management
- Administrators can customize the document lifecycle
- Assign a document lifecycle to the specified document typeWorkflow Initiation
- Manually initiate workflow
- Automatic flow workflow
Task push and completion
- Task push executor
- Reminder(E-mail、wechat、platform message)
Document library, fully personalized folder creation, stronger scalability
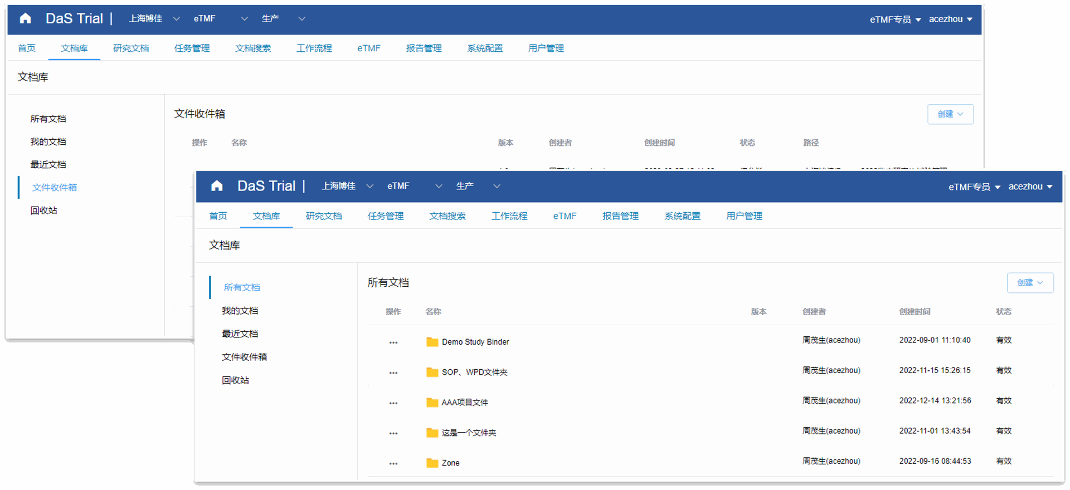
Interface structure more suitable for clinical investigators